Background: Acquired amegakaryocytic thrombocytopenic purpura (AATP) is a rare bone marrow disorder characterized by a marked reduction in megakaryocytes with otherwise normal hematopoiesis. Both humoral and cell-mediated suppression of megakaryocytopoeisis have been postulated as mechanisms causing AATP. Herein we report a case of a 67-year-old man diagnosed with AATP with a co-existent paroxysmal nocturnal hemoglobinuria (PNH) clone and review the literature on AATP, focusing on proposed etiologies for this rare condition.
Case: A 67-year-old man presented to clinic with a 1-week history easy bruising, petechiae and a platelet count of 6 x 109/L. He had a history of left elbow bursitis caused by S. pyogenes, treated with antibiotics 6-months prior to his presentation. His CBC was normal at that time. On assessment, HIV, HBV, HCV, and H. pylori serologies were negative; CMV and EBV serologies were positive for IgG and negative for IgM. ANA and RF were negative, and vitamin B12 level was normal. There was no hepatosplenomegaly on ultrasound. Bone marrow aspirate and biopsy demonstrated a normocellular marrow with severe megakaryocytic hypoplasia. Cytogenetics demonstrated normal male karyotype with loss of Y chromosome in 9/20 metaphases. Flow cytometry revealed a population of 3.99% GPI-deficient neutrophils by FLAER assay. Molecular testing for myeloid mutations and T-cell gene rearrangement is pending. The patient was initially treated with corticosteroids and IVIG, and showed no response with persistent isolated thrombocytopenia. He was managed with platelet transfusions which resulted in a normal platelet increment of 35 x 109/L 1-hour post-transfusion. A diagnosis of AATP was established and he was admitted for immunosuppressive therapy (IST) with ATG and cyclosporine.
Methods: We conducted a narrative review of the literature on AATP, searching MEDLINE and EMBASE for articles on AATP published in English between 1946 and 2020. Reference lists of selected articles were reviewed to identify additional cases. We extracted data on presentation, bone marrow findings (including cytogenetics, molecular genetics, and flow cytometry), treatment regimens and outcomes.
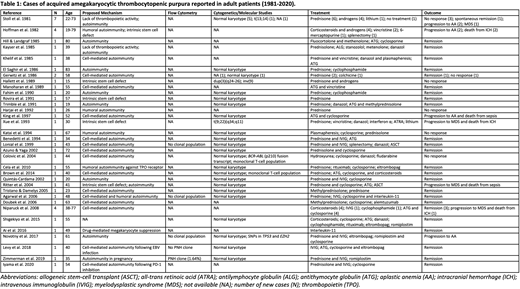
Results: We identified 47 cases of adult patients with thrombocytopenia attributed to AATP reported in the literature (Table 1). Three main mechanisms were proposed: (i) cell-mediated autoimmunity, (ii) humoral autoimmunity, and (iii) intrinsic stem cell defect. All three mechanisms were supported by in vitro studies, which demonstrated suppression of colony forming unit-megakaryocytes (CFU-M) by patients' T-lymphocytes (Gerwitz et al. 1986; Colovic et al. 2004) and serum (Hoffman et al. 1982) found to contain IgG antibodies inhibiting CFU-M formation, as well as intrinsic defects in CFU-M progenitor proliferation. Few studies reported cytogenetic abnormalities and only one documented molecular genetic testing. Response to IST was reported in several cases, most commonly ATG and cyclosporine. Four recent cases demonstrated remission following treatment with TPO agonists eltrombopag and romiplostim. Six cases progressed to aplastic anemia and 4 to myelodysplastic syndrome (MDS). Flow cytometry results were not reported in the majority of cases, and only 1 case reported coexistence of a PNH clone, identified in a pregnant patient with AATP (Zimmerman et al. 2019).
Discussion: AATP is defined as severe thrombocytopenia with bone marrow showing marked decrease or absence of megakaryocytes with preservation of other cell lineages. This broad definition encompasses a range of causes, and our review of the literature highlights the heterogenous nature of AATP which has several proposed mechanisms and a number of therapeutic options. The best evidence suggests that AATP is often secondary to T-cell-mediated suppression of megakaryocytopoeisis, which has been demonstrated by in vitro studies, and is supported in vivo by a case of AATP following PD-1 inhibition (Iyama et al. 2020), and frequent response of AATP to T-cell-directed IST. The co-existence of a PNH clone in our case lends further support to a T-cell-mediated autoimmune process, analogous to the mechanism described in aplastic anemia and hypoproliferative MDS. The application of molecular diagnostics may help to further elucidate the role of clonal hematopoiesis and intrinsic stem cell defects versus humoral and cell-mediated autoimmunity in AATP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.